For this reaction KMnO4 is reduced into MnO2. 2KMnO4+3Na2SO3+H2O→2MnO2+3Na2SO4+2KOH" in Chemistry if you're in doubt about the correctness of the answers or there's no answer, then try to use the smart search and find answers to...2MnO2 + 2H2SO4(конц.) = 2KMnO4 + 16HCl = 2MnCl2 + 2KCl + 5Cl2↑ + 8H2O. Ответ. Свернуть. 7) 3MnO2 + 4Al → 3Mn + 2Al2O3. Ответ. Свернуть.2KMnO4+3Na2SO3+H2O→2MnO2+3Na2SO4+2KOH. MissPhiladelphia MissPhiladelphia. The reducing agent would be the substance that is oxidized in the reaction or the one who loses electrons.13. Which substance is the reducing agent in the following reaction? e. An example of a good oxidizing agent is an alkali metal, such as Na. 16. Which of the following statements about electrochemical cells is true? a. Reduction occurs at the anode b. An element with a high love for...2KMnO4+3Na2SO3+H2O→2MnO2+3Na2SO4+2KOH. 2. The significance of category theory for philosophy 3. What is the deep. 1. Consider the overall reaction involving three elements as reactants, and a compound as the product.
Тренажер Задания 32 По Химии Марганца | Chemege.ru
So, the reaction between Mohr's salt and KMnO4 is a redox reaction in which oxidation and reduction takes place simultaneously. Potassium permanganate acts as self-indicator in this titration. Appearance of the end point can be detected by the colour change of KMnO4 from colourless to light...MnO4- + SO32- = MnO2 + SO42 When an equation is written in the molecular form the program will have issues balancing atoms in parcial equations of oxidation and reduction (Step 3.). This is avoided by writing the equation in the ionic form....reduce and also identify oxidising agent and reducing agent in the following reaction Fe2O3+3CO gives 2Fe+3CO2 3MnO2+4Al gives 3Mn+2Al2O3 Always remember the substance being oxidised is reducing agent and vice versa. SO2 is reduced and is oxidising agent.2 KMnO4 (aq)+3 Na2SO3 (aq)+H2O (l)→2 MnO2 (s)+3 Na2SO4 (aq)+2 KOH (aq). Appearance: Purplish-bronze-gray needles | magenta-rose in solution ; Dark purple crystals. Na2SO3 - Sodium sulfite.

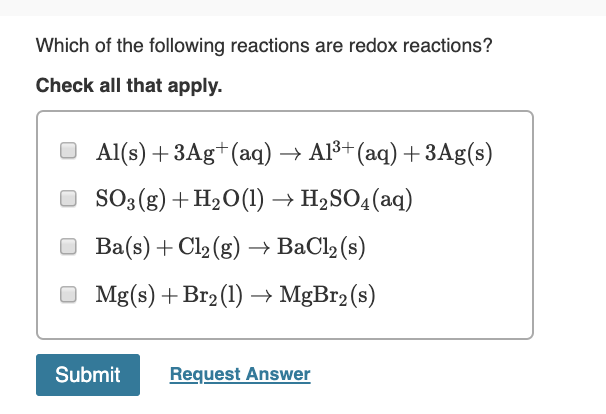
Which substance is the reducing agent in this reaction?
2KMnO4 + 3Na2 SO3 + H2 O → 2MnO2 + 3Na2 SO4 + 2KOH Reducing agent in the above reaction shown is : A. K+. B. Answer. Reducing agent in the above reaction shown is SO32− ion as S atom itself is oxidized. The oxidation number of S increases...agent so let's go ahead and write that here so sodium even though it it is being oxidized right it is the reducing agent it is allowing chlorine to be reduced by supplying these two electrons and chlorine alright chlorine by undergoing reduction it's taking the electrons from the two sodium atoms that...2KMnO4+3Na2SO3+H2O→2MnO2+3Na2SO4+2KOH In a reaction, the reducing agent is the element or compound that donates electron or the one tht loses electrons. For this reaction KMnO4 is reduced into MnO2.The substances which act as reducing agent and oxidizing agent in the given redox reactions have to be identified. The substance which accepts electrons in a redox reaction is known as oxidizing agent. Hydrogen peroxide, oxygen are examples of oxidizing agent.Now the two half reactions can be combined just like two algebraic equations, with the arrow serving as the equals sign. The same species on opposite Unless otherwise noted, it does not matter if you add H2O or OH− as a source of O atoms, although a reaction may specify acidic solution or basic...
you must depart a space at times. Else YA will "..." your text as you'll be able to see above
this
2 KMnO4 + 3 Na2SO3 + H2O → 2 MnO2 + 3 Na2SO4 + 2 KOH
no longer this
2KMnO4+3Na2SO3+H2O→2MnO2+3Na2SO4+2KOH
***********
anyway..
.. "reduction".. slightly actually manner "reduction in oxidation state"
.. "oxidation".. approach the opposite.. "increase in oxidation state"
and
.. "agents" are opposites
looking at the balanced equation.
.. all K, O, H and Na remained unchanged in oxidation states.. (you test this!)
and
.. Mn went from +7 in KMnO4 to +4 in MnO4
.. S went form +4 in Na2SO3 to +6 in Na2SO4
since
.. Mn went from +7 to +4.... Mn was REDUCED and is the OXIDIZING AGENT
.. S went from +Four to +6.. . ...S was once OXIDIZED and is the REDUCING AGENT
**********
now..
.. SOME instructors would say "S" is the reducing agent
.. SOME instructors would say "Na2SO3" is the reducing agent
I might probably say "S".. the keep away from the arguments
. (1) Na2SO3 is an ionic solid.. it's actually the SO3(2-) what was oxidized
. (2) is it the Na2SO3 or the Na2SO4 that was once "oxidized".. reactant or product
..... .or is it the SO3(-2) or SO4(-2) that is the "reducing agent"?
by way of pointing out "S" is the reducing agent, you avoid all that stuff.. however in this case since your teacher said.. "enter the formula".. I'm guessing she or he is of the "the entire compound not just the S" clan.
*********
final solution... . Na2SO3
Solved: Which Substance Is The Reducing Agent In This Reac
Solved: Which Substance Is The Reducing Agent In This Reac

Solved: Which Substance Is The Reducing Agent In This Reac

0 comments:
Post a Comment